|
|
|
|
|
|
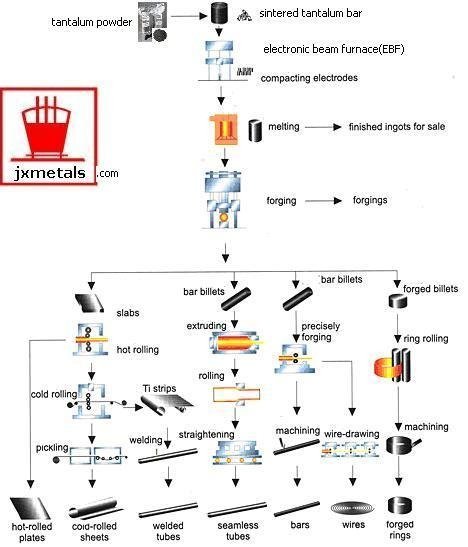
INTRODUCTION
Tantalum (formerly called tantalium), it is originated from Latin Tantalus sometimes called Tantale or Tantal Sonderwerkstoffe. Tantalum has a melting point of 2996°C and a density of 16.65 gm/cc, it is one of refractory metals (the most extensively used of these metals are tungsten, tantalum, molybdenum and columbium or niobium), most sheet-metal fabrication of tantalum can be welded to itself and to certain other metals by resistance welding, tungsten inert-gas (TIG) welding, and to itself by inert-gas arc welding. Electron-beam welding can also be used, particularly for joining to other metals. However, surfaces that are heated above 315°C during welding must be protected with an inert gas to prevent embrittlement.
Tantalum and tantalum alloys are midway between tungsten and molybdenum in density and melting points. Tantalum can be worked easily at room temperature. Tantalum's corrosion resistance is excellent in most acids and caustics as glass . Pure tantalum recrystallizes at approximately 2200°F (1204°C).
Principal applications for tantalum are in capacitor anodes, filaments, gettering devices, chemical-process equipment, and high-temperature aerospace engine components. Tantalum offers excellent "gettering" properties, making it popular in vacuum tubes to absorb products of out-gassing upon heat up of the tube components. It is also used to getter potential contaminants of niobium and its alloys as well as titanium during vacuum heat treating operations. Tantalum also provides good thermal conductivity that, combined with its corrosion resistance, has made it the ideal choice for heat exchangers for acid processing equipment. It is superior to the nickel-based alloys in both these categories.Tantalum also develops a stable oxide (Tantalum Pentoxide) that is useful in electronics industry applications.
Tantalum Alloys are used for specific applications:
· 97.5% Tantalum; 2.5% Tungsten(Tantalum 2.5% tungsten alloy)
This alloy is particularly useful in applications where low temperature strength is important along with high corrosion resistance and good formability, offering higher strength than pure tantalum while maintaining the fabricability-characteristics.
· 92.5% Tantalum 7.5% Tungsten(92.5/7.5 Ta/W tantalloy), it can be used as the regulator springs of the gas chlorinators.
· 90% Tantalum; 10% Tungsten(Tantalum 10% tungsten alloy) This alloy should be considered when high temperatures of up to 4500°F and high strength in a corrosive environment are required. The alloy has approximately twice the tensile strength of pure tantalum and yet retains tantalum's corrosion resistance and a good portion of tantalum's ductility.
Available milling materials: tantalium, tantal, tant, tantale,Sonderwerkstoffe

- TANTALUM ALLOY (Ta alloy)
- Tantalum alloy(Ta10%W, Ta-10W, Ta10W, 90% Tantalum 10% Tungsten / Wolfram) rod
4.0mm up to 95mm Diameter x random length,
90% tantalum, 10% tungsten, as per ASTM B365 - 92 R05255;
- Tantalum alloy(Ta2.5%W, Ta-2.5W, Ta2.5W, 97.5 % Tantalum 2.5 % Tungsten / Wolfram) rod
4.0mm up to 95mm max. x random length.
97.5% tantalum, 2.5% tungsten, as per ASTM B365 - 92 R05252;
- Tantalum alloy (Ta10%W, Ta-10W, Ta10W, 90% Tantalum 10% Tungsten / Wolfram) wire
0.15mm up to 3.0mm Diameter x coil at random length
90% tantalum, 10% tungsten, as per ASTM B 365 - 92 R05255;
- Tantalum alloy (Ta2.5%W, Ta-2.5W, Ta2.5W, 97.5 % Tantalum 2.5 % Tungsten / Wolfram) wire
0.15mm up to 3.0mm Diameter x coil at random length
97.5% tantalum, 2.5% tungsten, as per ASTM B 365 - 92 R05252;
- Tantalum alloy (Ta2.5%W, Ta-2.5W, Ta2.5W) Sheet
0.1mm up to 20mm thickness x Width up to 140mm x Length
Tantalum 97.5%, Tungsten 2.5%, as per ASTM B708 - 98 R05252;
- Tantalum alloy (Ta2.5W, Ta-2.5W, Ta2.5W) seamless tube
OD2.0 up to 38 mm Outer Diameter x 0.2mm up to 5 mm Wall thickness x Length 6 meters max.
Tantalum 97.5%, Tungsten 2.5%, as per ASTM B521- 98 UNS Grade R05252;

- TANTALUM
- Tantalum rod (ASTM B365 - 92 R05400)
4.0mm up to 95mm max. Diameter x random length;
- Tantalum wire (ASTM B 365 - 92 R05400 + ASTM F560)
0.15mm up to 3.0mm Diameter x coil at random length;
- Tantalum plate, tantalum sheet(ASTM B708 - 98 R05200)
0.2mm up to 1.0 mm Thickness x Width up to 500mm max. x Length,
1.0mm up to 20mm Thickness x Width up to 650mm max. x Length;
- Tantalum foil (ASTM B708 - 98 R05200)
0.025mm up to 0.05mm Thickness x Width up to 100mm max. x Length;
- Tantalum Strip (ASTM B708 - 98 R05200)
0.05mm up to 0.90mm Thickness x Width up to 130mm max. x Length in coil;
- Tantalum seamless tube(ASTM B521 - 98 UNS Grade R05200)
Tantalum capillary tube/pipe (ASTM B521 - 98 UNS Grade R05200)
1.0mm up to 2.0mm Outer Diameter x 0.2mm up to 0.35mm Wall thickness x Length 1 meters max.;
Tantalum seamless tube/pipe(ASTM B521 - 98 UNS Grade R05200)
2.0mm up to 38mm Outer Diameter x 0.2mm up to 5 mm Wall thickness x Length 6 meters max.;
- Tantalum sputtering target (purity 99.95%min, or 99.99%min).
6mm up to 20mm thickness x 600mm max. width x 1200mm max. length;
Tantalum and tantalum alloys has an excellent corrosion resistance in most acids and caustics as glass, e.g.
Tantalloy® 63 can resist Nitric Acid;
Tantalum can resist Hydrochloric Acid, Hypochlorites, Mixed Acids, Nitric Acid, Phosphoric Acid, Sodium Hydroxide;
Tantalum alloy can resist Mixed Acids;
Tantalum carbide can resist Hydrochloric Acid Nitric Acid, Phosphoric Acid;
Tantalum coatings can resit Phosphoric Acid;
Tantalum-tungsten alloy can resit Nitric Acid ;
Tantalum-tungsten alloys can resit Sodium Hydroxide ;
Tantiron® E can resit Nitric Acid ;
Tantiron® N can resit Nitric Acid ;
TaRe can resit Hydrochloric Acid ;
TaV can resit Hydrochloric Acid ;
TaW can resit Hydrochloric Acid ;
TaWHf can resit Hydrochloric Acid ;
TaWMo can resit Hydrochloric Acid ;
TaWNb can resit Hydrochloric Acid ;
TaWRe can resit Hydrochloric Acid ;
TaZr can resit Hydrochloric Acid ;
pure tantalum can resit Hydrochloric Acid, Nitric Acid;
comparing to that of tungsten and PTFE as the following :
Tungsten can resit Hydrochloric Acid, Hypochlorites, Mixed Acids, Nitric Acid, Phosphoric Acid, Sodium Hydroxide;
Tungsten carbide can resit Mixed Acids, Nitric Acid, Phosphoric Acid;
PTFE can resit Hydrochloric Acid, Hypochlorites, Mixed Acids, Nitric Acid, Phosphoric Acid, Sodium Hydroxide;
PTFE compound can resit Hydrochloric Acid;
PTFE yarn can resit Hydrochloric Acid;
Typical Applications for Tantalum
| Tantalum Product |
Application |
Technical Attributes/Benefits |
| Tantalum carbide |
Cemented Carbide Cutting tools |
Increased high temperature deformation, control of grain growth |
| Tantalum oxide (Tantalum Pentoxide) |
- Camera lenses
- X-ray film
- Ink jet printers |
- High index of refraction for lens compositions
- Yttrium tantalate phosphor reduces X-ray exposure and enhances image quality
- Wear resistance characteristics. Integrated capacitors in integrated circuits (ICs) |
| Tantalum powder |
Tantalum capacitors for electronic circuits in medical appliances such as hearing aids, pacemakers, also in airbag protection systems, ignition and motor control modules, GPS, ABS systems in automobiles, laptop computers, cellular phones, Playstation, video cameras, digital still cameras. |
High reliability characteristics and low failure rates, operation over a wide temperature range from –55 to +125°C, can withstand severe vibrational forces, small size per microfarad rating/electrical storage capability. |
| Tantalum sheets, Tantalum plates, Tantalum rods, Tantalum wires, Tantalum 2.5% tungsten alloy(Ta2.5W), Tantalum 7.5% tungsten alloy(Ta7.5W), Tantalum 10% tungsten alloy(Ta10W) |
- Tantalum Sputtering targets
- Chemical process equipment
- Cathodic protection systems for steel structures such as bridges, water tanks
- Prosthetic devices for humans – hips, plates in the skull, also mesh to repair bone removed after damage by cancer
- Suture clips
- Corrosion resistant fasteners, screws, nuts, bolts
- High temperature furnace parts.
- High temperature alloys(Ta10W alloy) for air and land based turbines (e.g. jet engines) |
- Applications of thin coatings of tantalum, tantalum oxide or nitride coatings to semi-conductors
- Superior corrosion resistance – equivalent in performance to glass. Attack by body fluids is non-existent. Melting point is 2996°C, but protective atmosphere or high vacuum required. Alloy compositions containing 2-11% tantalum offer high temperature reliability, resistance to corrosion by hot gases. |

Biocompatibility of Tantalum
by Robert J Harling
BSc(Hons) CBiol, MIBiol, DipRCPath, MRCPath, Eurotox Registered Toxicologist; April 2002
(The Report comes from Danfoss Tantalum Technologies.)
INTRODUCTION
This document reviews literature that presents information pertinent to the issue of tantalum’s biocompatibility. The information comes from the scientific literature, from extraction studies undertaken by Danfoss Technology Centre, and surface evaluation studies undertaken by The Danish Polymer Centre, Risø National Laboratory, Denmark.
SCIENTIFIC LITERATURE
(i) Physical Properties
Tantalum and its alloys retain significant mechanical properties up to 1000 °C. Tantalum is chemically stable, oxidising in air at 300 °C, and it has excellent corrosion resistance, being attacked only by strong acids and alkalis which hydrolyze to form hydrofluoric acid.
Tantalum symbol Ta
Atomic Number 73
Mean Atomic Weight 180.95
Periodic Table Grouping VB together with vanadium and niobium
Density 16.6 g.cm3
Melting Point 3000 °C
Despite being a reactive metal, (by periodic table location), tantalum is considered to be a noble material in practical terms.
(ii) Material Response
There is little published data relative to in vitro studies to predict in vivo degradation. Tantalum is covered by a very low solubility tantalum oxide film, over a wide range of pH and pO2 combinations which are reflective of biological situations. The tantalum/tantalum oxide equilibrium reaction is impossible to characterise directly due to the protective power of the oxide. In vivo corrosion release is very slight, there being no reports indicating local, systemic or remote site concentrations related to corrosion release. The most usual observation in both animals and clinical reports is the absence of visible corrosion or corrosion products. In a specific biocompatibility study Watari et al studied tantalum after implantation in the subcutaneous tissue of the abdominal region, and in the femoral bone marrow of rats for either 2 or 4 weeks.
No dissolution of the metal in soft tissues was detected using an x-ray scanning analytical microscope (XSAM), and no dissolution of the metal was detected in bone using electron probe microanalyzer elemental (EPMA) mapping procedures. The study concluded that tantalum had acceptable biocompatibility for use as a biomaterial. Where motion between implant and tissue are possible, then slight discolouration has been noted on some occasions. This is similar to the situation which occurs with titanium and titanium alloys, and is possible secondary to the removal of oxide particulates. Intake of tantalum and tantalum oxide produces very low levels of tantalum absorption from either the respiratory or gastrointestinal systems, again a reflection of the low solubility of the material. Tantalum clears promptly from lungs, airways and oesophagus in both animals and humans in the absence of respiratory disease.
(iii) Host response
Tantalum particles (10 to 50 µm) and pure titanium both cause no growth inhibition in human dermal fibroblast cultures. Other studies group tantalum with a number of other metals and alloys including stainless steel and pure titanium in relation to lack of biological effects. It is difficult to find standard data relating to the toxicological effects of tantalum. References indicate that there is no known human disease which is attributable to tantalum, that systemic poisonings in industrial situations are unknown, and that tantalum and tantalum compounds are not listed as presumptive or possible carcinogens. The oral LD50 for tantalum pentoxide in rats is quoted in one reference to be greater than 8 g/kg bodyweight. Where labelled tantalum has been injected into animal models only 15% is retained within the body, the balance being rapidly excreted. Forty percent of that which is retained within the body is retained within bone.
When tantalum is implanted as a tantalum foil, tantalum wire or tantalum mesh in soft tissues in either animals or humans, the main local tissue reponse is the formation of a thin, glistening membrane without any evidence of inflammation. This response has been characterised by loose and vascularised fibrous tissue with in some case the presence of an epithelium in contact with the implant. In work by Crochet et al an understanding of the pathological processes following implantation of tantalum stents into the femoral artery of sheep provides further evidence of the good biocompatibility displayed by tantalum based products. During the first four days after implantation a covering by non-organised throbi was noted. By fifteen days neointimal hyperplasia completely covered the stented arterial segment. This fibroblastic tissue showed no foreign body reaction. By 42 days collagen and myofibroblastic cells had progressively replaced the fibroblastic tissue indicative of a healing process. A similar reponse is seen with pure titanium, titanium alloys, zirconium, niobium and platinum upon implantation. In a specific biocompatibility study Watari et al studied tantalum after implantation in the subcutaneous tissue of the abdominal region, and in the femoral bone marrow of rats for either 2 or 4 weeks. No inflammatory response was observed around the implants and all were encapsulated with thin fibrous connective tissue. The study concluded that tantalum has sufficient biocompatibility for use as a biomaterial.
Early studies did report abscesses following cerebral apposition of tantalum in humans, however, infection has to considered as a potential reason rather than a tissue reponse to the implanted material. In addition some of the early clinical studies have to be questioned due to the source, purity, pre-operative cleaning and sterilisation processes used for the implanted tantalum. When implanted as tantalum foil, tantalum wire, tantalum rod or tantalum ball there are several reports that tantalum can be osteo-integrated. That is, direct apposition of bone is seen against the implant without an intervening soft tissue layer or capsule. It has been suggested that the reason for this is that, like titanium, tantalum has an electrically non-conductive surface oxide which does not denature proteins and thus permits osteo-integration. Work supporting this concept is presented by Zitter et al who describe an in vitro system for measuring current densities of metals used in implants. These measurements produce results which are in good agreement with results from in vivo biocompatibility studies. In their studies pure metals like titanium, niobium and tantalum showed the lowest current density values which correlates with these materials having high biocompatibility. The reason quoted for these materials having low current densities is the presence of a stable oxide layer on the base metals. The stable oxide layer prevents an exchange of electrons and thus any redox reaction. Hence the materials are bio-inert. Bobyn et al (1) utilised cylindrical implants of tantalum which were 75 to 80% porous, in a 52 week dog study where they were implanted into the femur. Bone ingrowth was clearly demonstrated in the study with high fixation strength occurring at an earlier time point with the porous tantalum implants. The report provides no indication of any adverse reactions during the procedures utilised. Work with alkali and heat treated tantalum by Kato et al describes the bone-bonding ability of tantalum in rabbit studies, and no histological effects indicative of an adverse reaction to the implants were noted in their study. Bobyn et al (2) studies the osseous tissue reponse to an implanted tantalum biomaterial in dogs with bilateral hip arthroplasties. Good bone growth was seen with the porous tantalum and histopathological examination confirmed the biocompatibility of the implants. In vitro work by Sharma et al demonstrated that the presence of the oxide layer on tantalum enhances the adsorption of protein at the interface. A mixture of proteins was used in the studies and these included albumin, globulin and fibrinogen. Adsorption of proteins onto the surface, rather than protein denaturation, will be one of the reasons for good biocompatibility results with tantalum implants.
In several studies tantalum has been acknowledged as being bio-inert and as such has been selected as a negative control in certain experimental situations. For example, Miller et al utilised tantalum as a negative control in a study where rats with tantalum implants were sampled for urine and plasma, and the samples tested for mutagenic activity using the Ames test. All results were negative. Chronically implanted stimulating electrodes for neural prostheses are being developed to alleviate neural deficits. In comparative work by Johnson et al the use of tantalum-tantalum oxide electrodes was investigated in brain implantation studies with cats. When removed at the end of the study all electrodes were loosely encapsulated by a fibrous sheath of dura-archnoid connective tissue. There was no tissue adhering to the electrode surface. Histologically there was a slightly thickened pia with a slight reaction of the subpial neuroglia and no neuronal reaction or inflammatory reaction in the cortex. The study concluded that the tantalum-tantalum oxide electrodes resulted in less tissue damage than with electrodes made from rhodium, platinum or carbon, and tantalum-tantalum oxide electrodes did not result in neurotoxic effects.
(iv) Clinical responses
Tantalum has been widely used in clinical applications for more than 50 years:
• as a radiographic marker for diagnostic purposes, due its high density
• as the material of choice for permanent implantation in bone, as osteomigration prevents migration
• as vascular clips, with the particular advantage that since tantalum is not ferromagnetic it is highly suited to MRI scanning
• in the repair of cranial defects - a United States of America medical material standard exists for tantalum in this application
• as a flexible stent to prevent arterial collapse
• as a stent to treat biliary and arteriovenous (haemodialyzer) fistular stenosis
• in fracture repair
• in dental applications
• in other miscellaneous applications
Aronson et al undertook a specific study of tantalum markers in radiography with pin and spherical markers being implanted into bony and soft tissues of rabbits and children. No macroscopic reaction was noted around the markers, those implanted into bone were firmly fixed exhibiting close contact with adjacent bone lamellae. Microscopic examination in rabbits showed no reaction or slight fibrosis in bone, and slight fibrosis, but no or only a minimal inflammatory response after 6 weeks. In the children, no inflammatory reaction and only slight fibrosis was present up to 48 weeks after insertion. The bio-inertness of tantalum was commented on in the conclusion of the paper.
(v) Extraction data
Extraction studies undertaken within the Danfoss technology Centre followed standard procedures (EN ISO 10993-12) to make extracts from various metals or metal combinations. Extracts were made utilising physiological saline and peanut oil at a temperature of 121 °C for one hour. Analysis of the extracts using ICP is presented below. The materials of particular interest are AISI 316 + Ta and Vitalium + Ta.
Results of analysis from physiological saline extracts
| |
Cobalt |
Chromium |
Copper |
Iron |
Manganese |
Molybdenum |
Nickel |
Lead |
Silicon |
Vanadium |
| |
Co (ppm) |
Cr (ppm) |
Cu(ppm) |
Fe(ppm) |
Mn(ppm) |
Mo(ppm) |
Ni(ppm) |
Pb(ppm) |
Si(ppm) |
V (ppm) |
| Blank |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,10 |
0,94 |
<0,10 |
| Tantalum |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,10 |
0,57 |
<0,10 |
| Vi |
0,24 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,10 |
0,82 |
<0,10 |
| AISI 316 |
<0,05 |
<0,05 |
<0,05 |
2,36 |
0,18 |
<0,05 |
0,06 |
<0,10 |
3,05 |
<0,10 |
| AISI 316+Ta |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,10 |
2,80 |
<0,10 |
| V + Ta |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,05 |
<0,10 |
3,64 |
<0,10 |
The report states that extraction of silicon from the glass experiment (as demonstrated by the blank value) means that data from this column is representative of extracted Si from the metals.
Results of analysis from peanut oil
| |
Cobalt |
Chromium |
Copper |
Iron |
Manganese |
Molybdenum |
Nickel |
Lead |
Vanadium |
| |
Co (ppm) |
Cr (ppm) |
Cu(ppm) |
Fe(ppm) |
Mn(ppm) |
Mo(ppm) |
Ni(ppm) |
Pb(ppm) |
V (ppm) |
| Blank |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<3,0 |
<2,0 |
<2,0 |
<0,5 |
| Tantalum |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<3,0 |
<2,0 |
<2,0 |
<0,5 |
| Vi |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<3,0 |
<2,0 |
<2,0 |
<0,5 |
| AISI 316 |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<3,0 |
<2,0 |
<2,0 |
<0,5 |
| AISI 316+Ta |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<3,0 |
<2,0 |
<2,0 |
<0,5 |
| V + Ta |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<0,5 |
<3,0 |
<2,0 |
<2,0 |
<0,5 |
The results of these studies show that for the AISI 316 + tantalum and the vitalium + tantalum none of the values from either the physiological saline or the peanut oil extractions exceeded the detection limits.
(vi) Surface analysis studies
In work undertaken at the Danish Polymer centre (part of the Risø National laboratory) studies were undertaken to analyse qualitatively the composition of both the outer surface and bulk material of tantalum coatings which had been applied to different substrates. Since the impurity levels in tantalum are so low, ToF-SIMS (Time-of-Flight Secondary Ion Mass Spectrometry) was utilised to analyse the samples. The results demonstrated that tantalum oxide was detected with similar or slightly higher intensity than tantalum during the initial part of the analysis, indicating that a thin layer of tantalum oxide exists. The results also demonstrate that the CVD-Ta coating on stainless steel has the lowest amount of impurities present, and this combination has an even better impurity profile than the tantalum reference.
CONCLUSION
Information available indicates tantalum is highly resistant to chemical attack and arouses very little adverse biological response in either the reduced or oxidised forms. Many studies demonstrate excellent biocompatibility in a variety of situations including, those applications involving bone surgery. Metals coated with tantalum and tantalum itself release nothing into extraction media during standardised procedures, and the surface analysis shows low impurity profiles.
Providing the tantalum used in the manufacture of the proposed medical devices meets the purity criteria there is no reason to undertake further biocompatibility studies in animals.
This report prepared by: Date: April 2002
Robert J Harling
BSc(Hons) CBiol, MIBiol, DipRCPath, MRCPath, Eurotox Registered Toxicologist
REFERENCES
Aronson,A.S., Jonsson,N. and Alberius,P. Tantalum markers in radiography. Sleletal Radiol. 1985, 14, 207-211.
Birkemose,N-R. Extraction report J.No. 2001734. Danfoss Technology Centre Internal Report 2001.
Black,J. Biological performance of tantalum. Clinical Materials 1994, 16, 167-173.
Bobyn,J.D., Stackpool,G.J., Hacking,S.A., Tanzer,M. and Krygier,J.J. Characteristics of bone ingrowth and interface mechanics of a new porous tanatalum biomaterial. J. Bone and Joint Surgery 1999, 81-B, No.5. (referred in text as Bobyn(1)).
Bobyn,J.D., Toh,K-K, Hacking,S.A., Tanzer,M. and Krygier,J.J. Tissue response to porous tantalum acetabular cups. J. of Arthroplasty 1999, 14, No.3, 347-354. (referred in text as Bobyn(2)).
Crochet,D., Grossetete,R., Bach-Lijour,B., Sagan,C., Lecomte,E., Leurent,B., Brunel,P. and Le Nihouannen,J-C. Plasma treatment effects on the tantalum strecker stent implanted in femoral arteries of sheep. Cardiovasc. Intervent. Radiol. 1994, 17, 285-291.
Johnson,P.F., Bernstein,J.J., Hunter,G., Dawson,W.W. and Hench,L.L. An in vitro and in vivo analysis of anodized tantalum capacitive electrodes: corrosion response, physiology and histology. J. Biomed. Mater. Res. 1977, 11, 637-656.
Kato,H., Nakamura,T., Nishiguchi,S., Matsusue,Y., Kobayashi,M., Miyazaki,T., Kim,H-M. and Kokubo,T. Bonding of alkali- and heat-treated tantalum implants to bone. J. Biomed. Mater. Res. 2000, 53, 28-35.
Matsuno,H., Yokoyama,A., Watari,F., Uo,M. and Kawasaki,T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253-1262.
Miller,A.C., Fuciarelli,A.F., Jackson,W.E., Ejnik,E.J., Emond,C., Strocko,S., Hogan,J., Page,N. and Pellmar,T. Urinary and serum mutagenicity studies with rats implanted with depleted uranium or tantalum pellets. Mutagenesis 1998, 13, No.6, 643-648.
Sharma,C.P. and Paul,W. Protein interactionwith tantalum: changes with oxide layer and hydroxyapatite at the surface interface. J. Biomed. Mater. Res. 1992, 26, 1179-1184.
Wei,J. Analysis Report: ToF-SIMS characterisation of Ta samples. Danish Polymer Centre, Risø National Laboratory Project No: COMF/ 2001.
Zitter,H. and Plenk Jr,H. The electrochemical behaviour of metallic implant materials as an indicator of their biocompatibility. J. Biomed. Mater. Res. 1987, 21, 881-896.
|
|
|
|
|
|
|
|